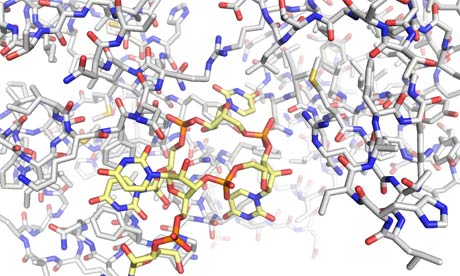
 N-terminal domain of La complexed with AUUUU RNA. Illustration: Stephen Curry
N-terminal domain of La complexed with AUUUU RNA. Illustration: Stephen Curry
Please don't be alarmed gentle reader, but I am here to tell you that you are an abominable monster. And I have made it my job, like an inverse Dr Frankenstein, to dismantle you. The work is exacting, but we have to find out what makes you tick, my fearsome friend.
This news may come as a shock, but if you don't believe me, take a look above.
Appalled at yourself? You should be. Your complexity is monstrous. And that is just one molecule plucked from among thousands in your body. It goes by the disarmingly simple name of La.
If you have ever peered into the crowded innards of a computer and recoiled in horror or wonder (the two are not so different), you will have some small sense of the task of working out the secrets of life. For the intricacies found in human artefacts cannot compare to the morass of molecules within each one of the trillions of cells in your body. Somehow, this crazy jumble of proteins, fats, carbohydrates and nucleic acids (DNA and its sibling, RNA) is keeping you alive.
Fortunately, we scientists are the monster's friend. And we are a dogged lot, not put off by the unnerving confusion of living things, though we often have to focus on the small details to make any headway.
My particular task, as a structural biologist, is to uncover the atomic architecture – seen in the image above – of the proteins and other molecules that work together inside you to sustain life. It is slow, painstaking work. I was reminded of this at a conference last week when I stood up to tell the audience about our research into the structure of La, which had started more than 10 years ago.
La caught my eye back then because of reports of its seizure by some viruses, such as hepatitis C virus, which force this innocent protein to co-operate with infection. We wanted to find out how that happens and, at the same time, to learn more about how the protein operates in healthy cells.
La is a tiny cog in the apparatus of life but has an important role in the process of translation that uses the information in our genes to make the proteins, the molecular contraptions that keep your cells alive and communicating with one another.
The mechanism of translation uses molecules of RNA – called tRNA – that are directly involved in the conversion of the DNA sequence of your genes into the chains of amino acids that fold into working proteins. The job of La is to ensure that these tRNA molecules are themselves built correctly. During tRNA synthesis, La holds on to one end the molecule to prevent it becoming damaged by accidental encounters with rogue proteins in the cell.
All of this we know by indirect methods, feeling blindly with measurements of the strength of the interaction between the protein and the RNA. But humans are visual creatures. To understand exactly how La embraces a tRNA molecule and how it might be ensnared by viral RNA, we have to see the structure of the protein.
Our work with La started by transferring the DNA for its gene into bacteria that are easily cultured in litres of nutritious soup. This genetic trick made it relatively easy to produce large quantities of La protein in the lab, entire milligrams of the stuff.
But we then had to spend a long time poking and dissecting the protein, testing its solubility, checking that it was folded properly, cutting and trimming to find a manageable fragment that might be induced to line up in orderly ranks to form a crystal, the shining jewel we needed to reveal the structure. After many trials and errors (and the distractions of securing funding and teaching duties), tiny crystals emerged from our experiments. They were 100th of a millimetre thick.
At the Diamond synchrotron near Oxford we took measurements as the crystals scattered the high-energy beam of X-rays in multiple directions, producing patterns of spots on the detector. Thanks to the mathematical techniques developed in the19th century by Joseph Fourier (who was grappling with the unrelated problem of heat flow – the connections in science are always unpredictable), we were able to use the spot pattern to calculate the three-dimensional structure of La, in all its atomic glory.
It took years of work, but finally we could unveil how part of La (our fragment is just half of the protein) grapples with one end of a tRNA molecule.
We have shown how two parts of two of the cogs of life fit together. In the grand scheme of things, it is just one small step forward, but it is nevertheless an important step, one that shows how the protein recognises – by feel, not sight – and protects the tRNA. Thus ensuring that the machinery of life keeps ticking over.
This long story is still incomplete. There are the remaining portions of La and the tRNA to consider. How do they contribute to the protective embrace between these two molecules? And is this embrace the same forceful grip by which the RNA of hepatitis C virus compels La to sabotage liver cells? To tackle these questions we've got to spend more years in the lab.
But just for a moment, let us pause to admire the horrific complexity of your La molecule in action by rendering the protein and RNA chains as friendly coils:
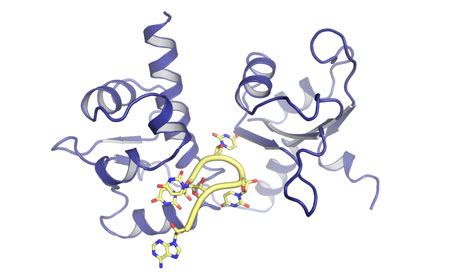
You may be monstrous, dear reader, but I never said you were ugly.
Stephen Curry is a professor of structural biology at Imperial College and writes a regular blog at Reciprocal Space. His work on La is a long-standing collaboration with Dr Maria Conte at King's College London
